Research Interests
Metabolic Engineering, Synthetic biology, Microbial Co-culture Engineering, Biosensing
Applied Microbiology, Natural Product BiosynthesisResearch Overview
| Our research aims at developing robust biological systems
for high-efficiency biosynthesis of various value-added compounds,
including commodity chemicals, specialty chemicals, nutraceuticals, cosmetics, and
pharmaceuticals. |
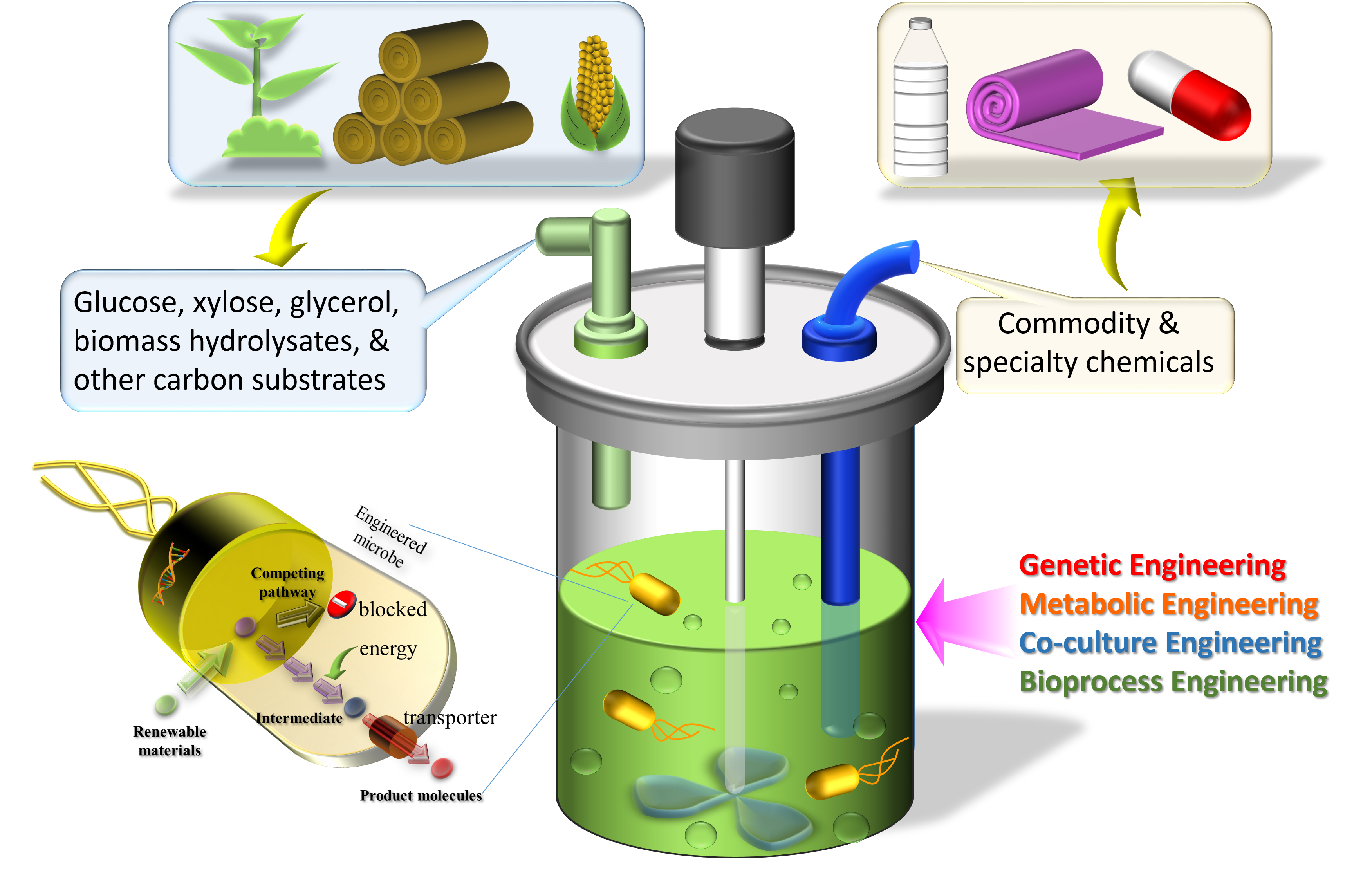 Bioproduction of commodity chemicals
Our group is dedicated to creating novel engineering tools and methods
for construction of robust microbial strains which are able to
efficiently produce molecules with high industrial values. To this end,
we engineer microorganism's genetic and metabolic setup in order to
create desired microbial factories to generate the target products. In
particular, our study focuses on converting renewable feed stocks, such
as sugars and glycerol, to make the desired products. Metabolic
engineering and process engineering approaches are employed to establish
and optimize the production process to achieve high titers,
productivities and yields.
Biosynthesis of complex compounds
The emerging technologies and approaches in bioengineering
offer unprecedented opportunities to modify and engineer microbes in
order to suit the needs of biosynthesis of complex molecules with
various biological values and applications. Our research utilizes
current advancement in bioengineering and biotechnology to explore the
potential of engineered microbial biosynthesis system. In
particular, we are interested in building novel biosynthesis
pathways leading to formation of new nutraceutical and
pharmaceutical molecules or optimizing the
identified pathways for improving the biosynthesis performance. In
addition, the microbial co-culture engineering strategies (see below)
are utilized for construction of platform biocatalytic systems that
consolidate the biosynthetic power of different microbial strains for
the purpose of making compounds hard to biosynthesize by traditional
methods. Our approach provides a new perspective to pursue discovery of
novel pharmaceutical molecules, such as antibiotics, and re-programming
of the existing biosynthetic routes that suffer from sub-optimal
bioproduction performance.
|
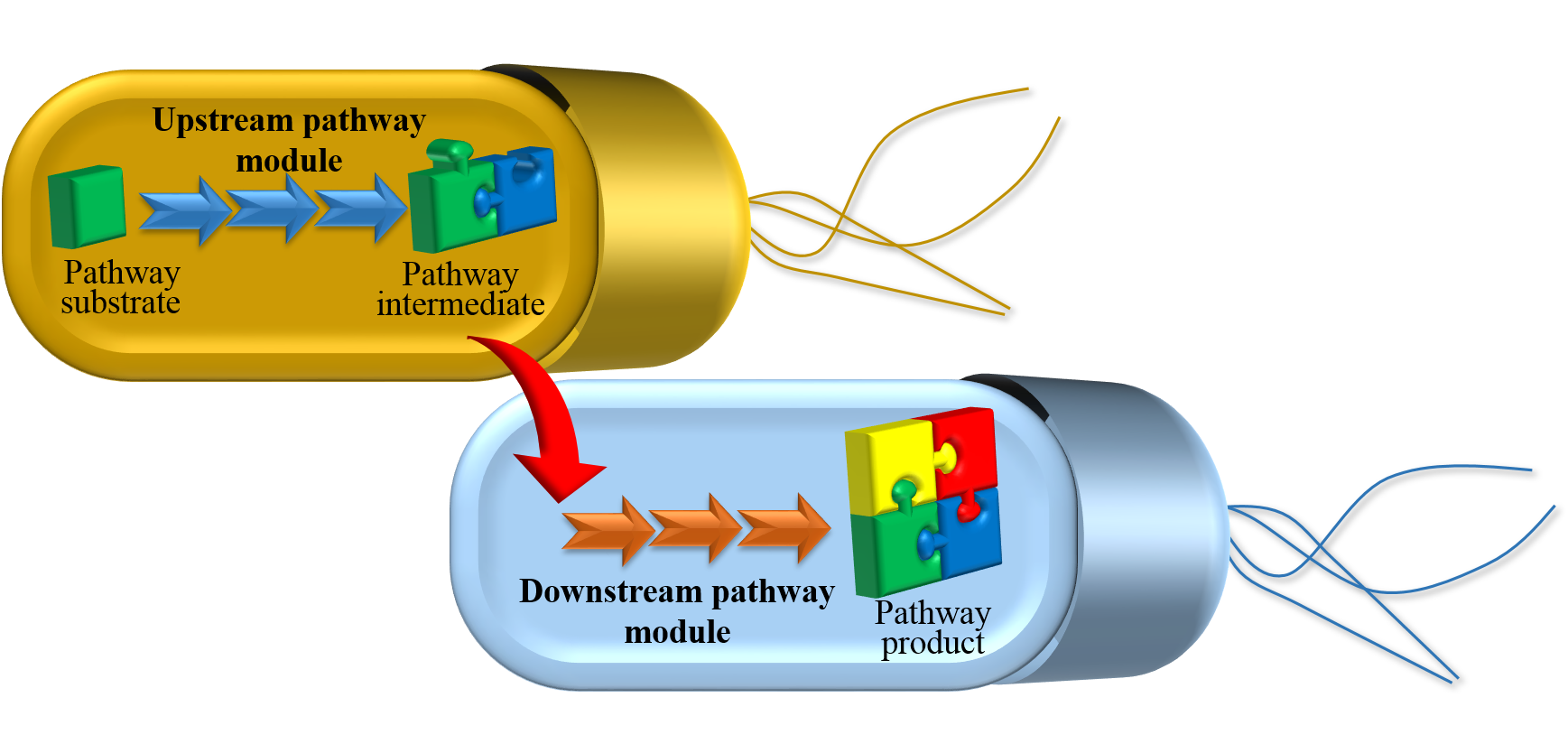 Microbial co-culture engineering
In addition to making specific products, we are also
interesed in developing novel methodologies and platform technqiues
that are generally applicable for biosynthesis of a wide range of
bioproducts. Specifically, we study how to use a consortium of
separate microbial cells (co-culture), rather than one
sinlge cell (monoculture) to
accommodate complicated biosynthetic pathways. Such a
modularization of biosynthetic task between multiple strains offers
several important advantages, such as metabolic burden reduction,
increased cellular enviroment diversity for enzyme activity
optimization, minimization of undesired cross-talk between different
pathway modules, flexible balancing between upstream and downstream
biosynthetic strength. Metabolic engineering and process engineering
approaches are utilized to ensure the stability of robustness of the
engineered co-culture systems. We have developed the microbial
co-cultures that signficantly improved the bioproduction
performance for a variety of biochemicals with varying chemical
structures and properties. Efforts are being made towards applying the
microbial co-culture engineering strategies for efficient
biosynthesis of highly complex and yet highly valuable compounds such
as nutraceuticals and pharmaceuticals.
Biosensing
Metabolite
biosensors are functional proteins, RNA or other biomolecules that can recognize
specific metabolites and accordingly regulate metabolic activities
such as gene expression. We study how to design and construct
biosensor-based metabolic regulation systems in selected microbes. Such systems are able to self-tune key genes' expression
according to target biomolecules' concentration change. As a result of
the dynamic sensing and regulation, the metabolic resources can be
rationally allocated to satisfy the need of both central metabolism and
the biosynthetic pathway of target products. Subsequetnly, we can
achieve the balance between cell growth and product
formation for biosynthesis optimization. To this end, we explore
innovative engineering strategies to maximize the biosensing and
regulation capbilities in the selected micorbial hosts to serve the need of
microbial bioproduction.
|
© Copyright 2021 the Zhang Lab
 |